



- Partners
- …
- Partners



- Partners
- …
- Partners

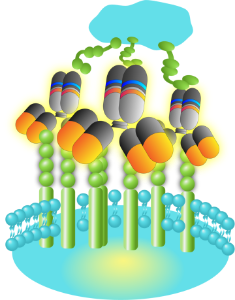
Immune-oncology Agonistic Antibodies
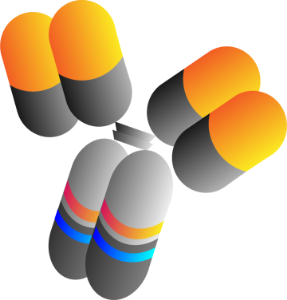
Cross-linking (xLinkTM)Synthetic Biology
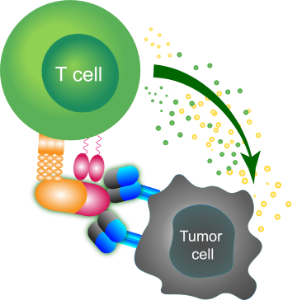
Tumor-targeted Immune Activation
Clinical Development Candidates
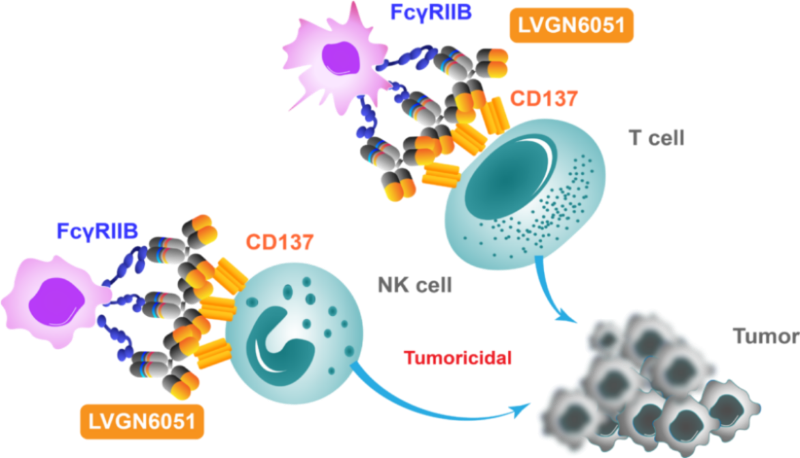
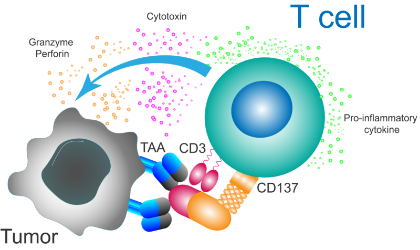
Next generation CD137/4-1BB agonistic monoclonal antibody
LVGN6051 is designed to stimulate the proliferation and cytotoxicity of antitumor immune cells. Optimal efficacy and safety profile are achieved by the crosslinking dependent CD137 agonistic activity through FcγRIIB.
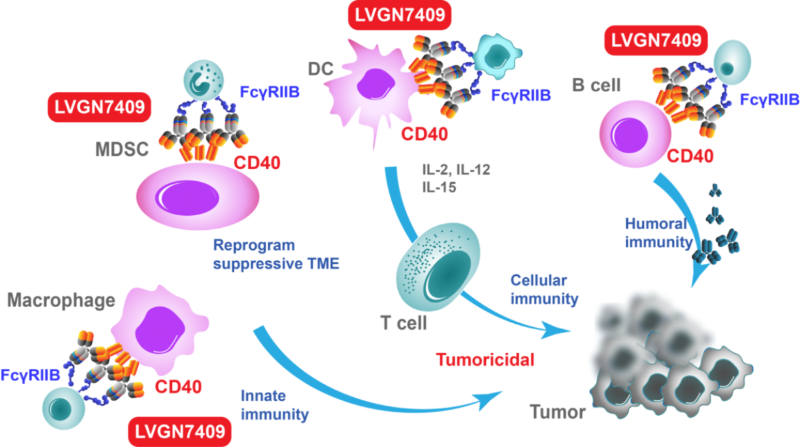
Next generation CD40 agonistic monoclonal antibody
CD40 activation by LVGN7409 promotes tumor antigen presentation and induction of cancer-fighting T cells, and "warm up" the immune-"cold" tumor microenvironment. Unique structure and functionality of LVGN7409 permits its higher tolerable dosage and broader clinical utility.
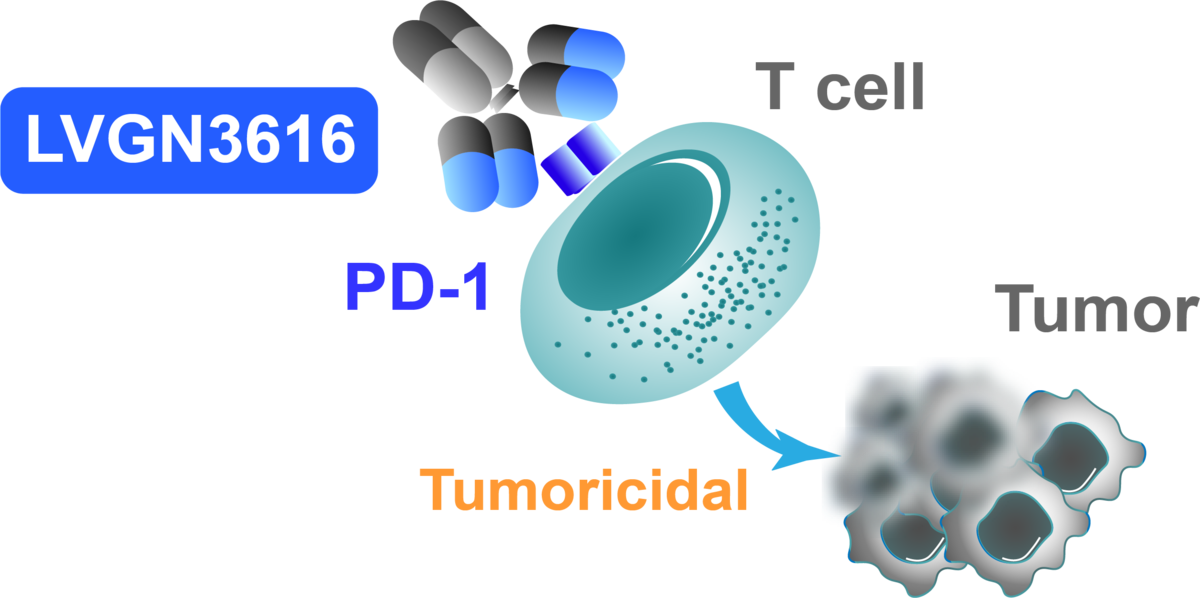
Proprietary PD-1 blocking monoclonal antibody
LVGN3616 blocks PD-1 pathway to unleash the "brake" of the immune responses against cancer.
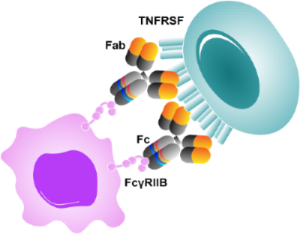
Lyvgen xLinkAb platform
xLinkAb Avidity Cross-linking Synthetic Biology
- Cross-linking monoclonal antibodies
- Target activation dependent on FcγRIIB engagement
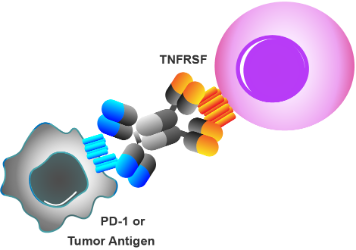
- Cross-linking dependent bi-specific antibodies
- Target activation dependent on binding to another antigen
- Cross-linking multi-specific antibodies
- New generation CD3 T cell engager with co-stimulation
- IgG-like design
Lyvgen Shanghai
Suite 317, Building 1, 351 Guoshoujing Road
Lyvgen Suzhou
Room 137, Building C36, 188 Dongping Street
Inquiries
General ------------------------------ ---Business Development----------------------------Employment--------------------------------------Clinical
info@lyvgen.com -----------------1 bd@lyvgen.com ------------ ----- careers@lyvgen.com ------------------ clinical@lyvgen.com
Phone +86 021-50663350
Copyright © 2022 LYVGEN BIOPHARMA. All Rights Reserved. Lyvgen website is designed to provide general information about the subject matter presented. The information presented does not, and is not intended to, provide medical advice.






 沪公网安备 31011502015346号
沪公网安备 31011502015346号