



- Partners
- …
- Partners



- Partners
- …
- Partners

News
Dr. C. Hubert Chan, Chief Medical Officer of Lyvgen Biopharma:
Innovation in Clinical Development for the Breakthrough Cancer Immunotherapy of Next-Generation IO Agonistic Antibodies
Lyvgen Biopharma, a biotech company focused on developing innovative immuno-oncology therapies, announced the appointment of Hubert Chan, M.D., Ph.D., as Senior Vice President and Chief Medical Officer. Dr. Chan oversees the clinical development of Lyvgen's lead candidates LVGN6051 (a new generation of CD137 or 4-1BB agonistic monoclonal antibody (mAb)) and LVGN7409 (a new generation of CD40 agonistic mAb), as well as other novel IO agonistic pipelines. Dr. Chan shared a recent exciting news of Lyvgen clinical program that the first patient was dosed in the phase I clinical trial of LVGN7409 (CD40 agonistic mAb) and LVGN3616 (PD-1 blocker mAb) combination therapy for the treatment of patients with advanced relapsed cancer. Both of the antibodies are in-house developed by Lyvgen. This clinical milestone is a major achievement for Lyvgen, and is expected to bring new treatment options to more patients.

Dr. C. Hubert Chan
Dr. C. Hubert Chan is a board-certified physician in medical oncology and internal medicine. He has been engaged in clinical practice and research for more than 20 years and participated as a clinical investigator in many Phase I to III clinical studies of chemotherapeutics, small molecules, and biological drugs. Entering the biopharmaceutical industry in 2016, he successfully provided the leading role of clinical development for pivotal studies and contributed to the regulatory approval of two anti-cancer agents in both FDA and EMA. Dr. Chan obtained his Ph.D. in Pathobiology from University of Minnesota and his M.D. from Chung Shan Medical University. He also received postdoctoral training at the prestigious Dr. Levy’s laboratory at Stanford University. Before joining Lyvgen, Dr. Chan worked at National Cancer Research Institute, Veteran General Hospital – Taipei, Chang Gung Memorial Hospital, PharmaEngine, Inc., and Shanghai Henlius Biotech. He leads a talented clinical team to advance the clinical development of Lyvgen’s innovative onco-immunotherapeutic agonistic antibodies.
Cancer immunotherapy (IO) is led by PD-1 and PD-L1 antibodies over the past few years. Since there are still majority of patients who cannot benefit from PD-(L)1 treatment, new drugs and treatment options are critically needed. LVGN7409, a CD40 agonistic mAb, and LVGN6051, a 4-1BB agonistic mAb, are currently in clinical phase and are expected to lead to new breakthroughs in the IO agonistic antibodies filed. What are the expected clinical applications of Lyvgen products? What specific clinical needs can be met? We invite Dr. C. Hubert Chan, Chief Medical Officer of Lyvgen Biopharma, to share his views and insights.
Q: Could you please tell us about your personal experience and why you chose to join Lyvgen as Chief Medical Officer?
A: I got my Ph.D. in Pathobiology from University of Minnesota and my M.D. from Chung Shan Medical University. I received postdoctoral training with Dr. Levy at Stanford University. After that, I returned to Taiwan and engaged as an oncologist for over 20 years. During this period, I have accumulated rich experience in clinical treatment and participated in several clinical trials of new tumor drugs, including chemotherapy drugs, low molecular, high molecular biological drugs from phase I to III. As a physician, the help I could offer to the patients was limited because of the lack of efficient treatment or the consultant time limit. Instead, as CMO at Lyvgen, I have the opportunity to bring more hopes to more patients. The decision of joining Lyvgen came from my confidence in Lyvgen's technology, its deep engagement in the R&D of IO agonistic antibodies. I believe that Lyvgen innovative agonistic antibodies can really help cancer patients. By leveraging my experience in oncology medicine and drug discovery, I am leading the team to practice innovation at the clinical development stage, which is just as much as if not more exciting and creative than at the preclinical R&D stage.
Q: Can you introduce the mechanism of two Lyvgen clinical candidates LVGN6051 and LVGN7409? And their current clinical development status?
A: LVGN6051 is an agonistic mAb targeting CD137 (4-1BB), which activates CD137-expressing T cells dominantly and other lymphoid cells, promotes cytokine release and cytotoxic activity, and enhances the function of tumor-infiltrating and cytolytic CD8+ T cells to achieve anti-tumor effect. LVGN7409 is an agonistic mAb targeting CD40, which activates CD40-expressing antigen-presenting cells (especially DC) to promote antigen presentation. It also can reverses the immunosuppression of macrophages to induce and drive the cytotoxicity of T cells to achieve anti-tumor effects. The Fc domains of LVGN6051 and LVGN7409 have been engineered to selectively bind to FcγRIIB, and can effectively activate CD137 or CD40 dependent on Fc-FcγRIIB cross-linking in tumor microenvironment, resulting in strong efficacy and better safety.
The clinical studies of LVGN7409 and LVGN6051 are progressing well with very encouraging data. As the monotherapy, both LVGN7409 and LVGN6051 show promising best-in-class profile of safety and efficacy. Their combination with PD-1 blocking antibodies are being testing in several selected indications. In addition, the combination of LVGN6051 and Anlotinib (VEGFR-TKI) for the treatment of sarcoma has been approved in China and the first-in-patient dosing is expected soon.
Q: Dose Lvygen have any new immune-oncology agonistic antibody candidates entering the clinic soon? What’s your prediction of the potential clinical breakthroughs of Lyvgen in the future?
A: Besides the three products that have entered the clinical stage, the R&D pipeline of the company is very rich, covering all stages of the tumor immunity process. In addition to CD40 and CD137, drugs targeting OX40 and GITR are also under development as agonistic monoclonal antibodies. Lvygen also has rich experience in the design and manufacture of multi-specific antibodies. By increasing the antigen binding sites, drugs can be targeted to the local tumor area, thus increasing the killing effect on target cells and the safety of treatment. For example, we have IND-enabling stage candidates bi-specifically targeting PD-1/CD137, B7H3/CD40 and PD-1/CD40, or multi-specifically targeting tumor antigen/CD3/CD137 as 3rd generation T cell engagers.
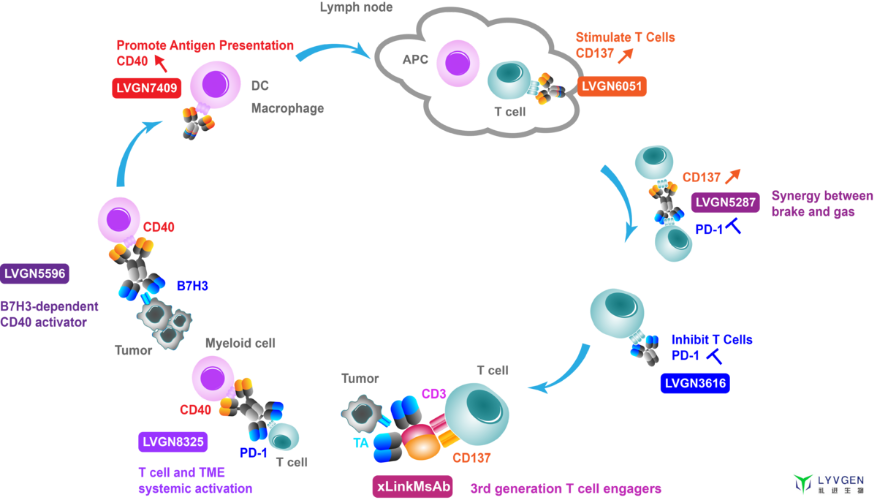
Clinical benefits for patients must be proven through rigorous clinical studies. There are no shortcuts. Lyvgen’s focus on immune agonists is widely applicable for most cancer indications. Our xLinkAb platform helps achieve the best balance between safety and efficacy, which can greatly increase the chances of success. The advantages of agonistic antibodies have been validated in several preclinical and clinical studies. At present, the agonistic antibody field is still young, but developing rapidly with huge potential as a hot field of cancer immunotherapy. Our strength and long-term engagement in IO agonistic antibodies are returning primary promising clinical results. I’m quite optimist about Lyvgen’s future and the realization of clinical benefit for patients. The potential clinical breakthroughs of our candidates will be benefit-cost effective drugs for a large panel of cancer indications and stages.
Interviewer: Lyvgen PR team
About Lyvgen
Lyvgen is an innovative biotech company focused on developing novel therapies for cancer. Its proprietary xLinkAb functional platform generated agonistic antibodies with tumour-localized immunostimulatory activities by balancing multiple functions of candidate antibodies, which has helped to generate a number of drug candidates, including LVGN7409 (CD40 agonist mAb) ,LVGN6051 (4-1BB agonist mAb) and LVGN3616(PD-1 antibody), presently in clinical development. The phase 1 clinical trial of LVGN7409 has been advanced in China and the United States, and preliminary clinical breakthrough safety and activity data have been obtained. The combined PD-1 antibody study is under way. LVGN6051 has demonstrated safety and activity and is being tested in a phase 1B /2 trial in the United States with Merck's PD-1 inhibitor Keytruda® (pembrolizumab) in adults with advanced tumors. LVGN6051 has also been successfully approved in Phase 1B /2 clinical trials in China in combination with small molecule inhibitors of vascular endothelial growth factor receptor, and clinical trials are being actively carried out.
For more information, visit www.lyvgen.com


 沪公网安备 31011502015346号
沪公网安备 31011502015346号