



- Partners
- …
- Partners



- Partners
- …
- Partners

LVGN7409
Anti-CD40 agonistic monoclonal antibody
Clinical trials
CD40 Agonist and PD-1 Inhibitor in HNSCC
NCT06159621
VIEW TRIAL
Phase 1 Trial of LVGN7409 (CD40 Agonist Antibody) as Single Agent and Combination Therapies in Advanced or Metastatic Malignancy
NCT04635995
VIEW TRIAL
Study of LVGN7409 (CD40 Agonist Antibody) in Locally Advanced, Metastatic or Recurrent/Refractory Malignancy
NCT05152212
VIEW TRIAL
Study of LVGN3616 and LVGN6051±LVGN7409 in Combination With Nab-Paclitaxel or Bevacizumab and Cyclophosphamide in Metastatic Solid Tumors
NCT05075993
VIEW TRIAL
More about LVGN7409
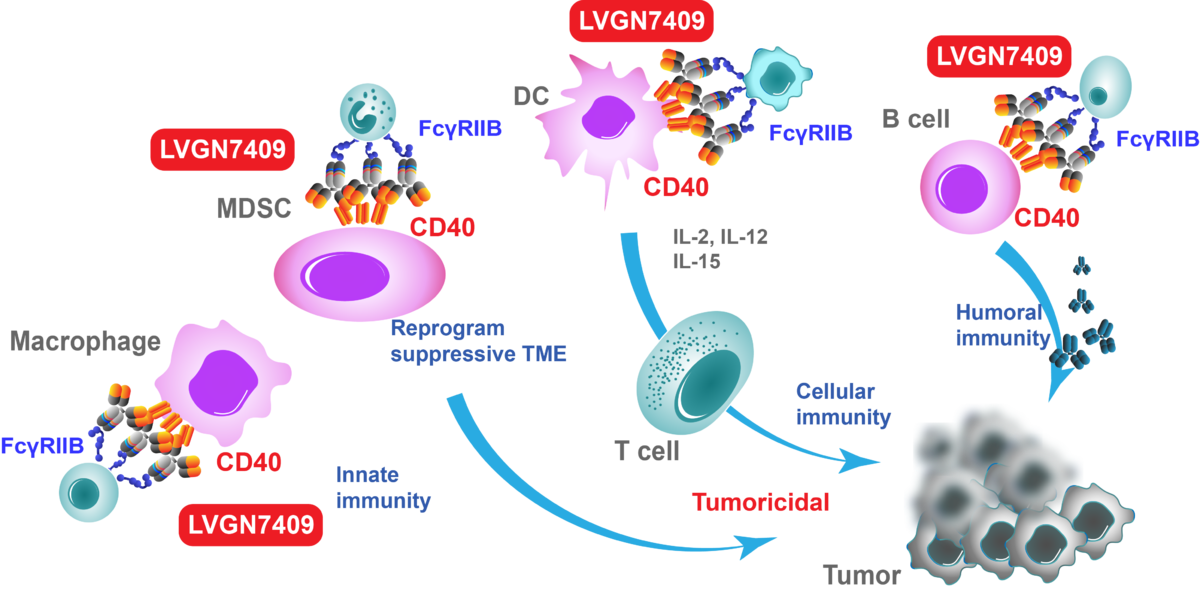
CD40 is a costimulatory protein found on antigen-presenting cells (APCs) and is required for their activation. The binding of CD154 (CD40L) on T cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.
Similar to the endogenous CD40 ligand (CD40L or CD154), CD40 agonistic antibody LVGN7409 binds to CD40 on a variety of immune cell types. This triggers the cellular proliferation and activation of antigen-presenting cells (APCs), and activates B-cells, and effector and memory T-cells. This results in an enhanced immune response against tumor cells. Evidence to date suggests that CD40 activation is a critical and nonredundant mechanism to convert so-called cold tumors to hot ones (with prominent tumor infiltration of T cells), sensitizing them to checkpoint inhibition.
Based on different expression profile and anti-tumor activity mechanism between CD40 and other T-cell activating drugs, anti-CD40 antibody is an excellent candidate for combination therapy with anti-PD-1, anti-PD-L1 or anti-CTLA4 antibodies etc.
For more scientific rationale, please visit Cancer Immunotherapy and xLink MAb.
Our ProgressBased on Phase I trials of LVGN7409 as monotherapy and in combination with PD-1 antibodies in the USA and China, in adult patients with advanced malignancies, we observed promising primary safety and efficacy resutls (ASCO 2022).
We are conducting studies of LVGN7409 in combination in Metastatic Solid Tumors (NCT05075993 ,NCT06159621)
If you as a patient or caregiver wish to participate in this study, please send us a messageLyvgen website is designed to provide general information about the subject matter presented. The information presented does not, and is not intended to, provide medical advice.
Lyvgen Biopharma Website 礼进生物 英文官网
Lyvgen is a biotech company focused on developing novel therapies for cancer. Lyvgen’s xLinkAb™ functional platform creates agonist antibodies (Abs) with tumor-localized immunostimulatory activities by balancing multiple functions of candidate Abs.
https://user-assets.sxlcdn.com/images/676507/FiTA8WK-FQRyXrQDyd-HDkY3tpfI.png?imageMogr2/strip/auto-orient/thumbnail/1200x630>/format/png

 沪公网安备 31011502015346号
沪公网安备 31011502015346号