



- Partners
- …
- Partners



- Partners
- …
- Partners

News
Lyvgen Biopharma Announces Publication of “Antibody-Targeted TNFRSF Activation for Cancer Immunotherapy: The Role of FcγRIIB Cross-Linking” in Frontiers in Pharmacology
July 5, 2022 – Lyvgen announced publication of a review under the research topic of Targeting TNF/TNFR Signaling Pathways in Frontiers in Pharmacology, the second most-cited journal in its field advances access to pharmacological discoveries to prevent and treat human disease.
The review entitled “Antibody-Targeted TNFRSF Activation for Cancer Immunotherapy: The Role of FcγRIIB Cross-Linking” summarizes the biological function of Tumor Necrosis Factor Receptor Superfamily (TNFRSF) members, the landscape of TNFRSF agonistic clinical antibody candidates, and the important regulation role of FcγRIIB cross-linking for the antitumor activities of the therapeutic agonists. The authors expand the rationale and relative evidences of Lyvgen’s xLinkAb platform technology for developing tumor microenvironment avidity-driven TNFRSF agonistic antibodies. This work was published in collaboration with Oncology Bio-informatics expert, Professor Guanglei Zhuang, and his team at the Key Laboratory of Oncogenes and Related Genes, Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, Shanghai Cancer Institute, Shanghai Jiao Tong University School of Medicine, China.
Through the publication of this review, Lyvgen shared its scientific rationale and success in innovating TNFRSF agonistic antibodies, including two clinical candidates LVGN7409 (CD40 mAb) and LVGN6051 (4-1BB/CD137 mAb). Based on this, Lyvgen hopes to carry out more in-depth exchanges and collaborations with collaborators and partners from academia, industry, and clinical institutes, and jointly realize the clinical benefit of agonistic antibodies for patients as soon as possible.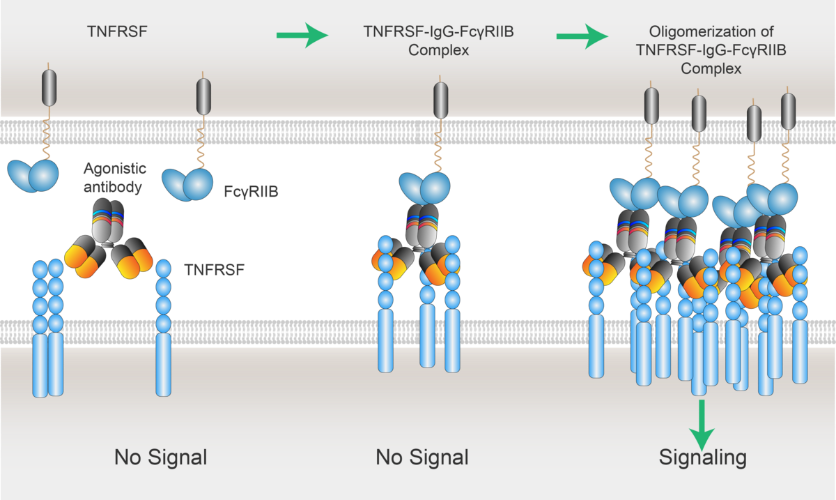
xLinkAbTM: The role of FcγRIIB in TNFRSF-IgG-FcγRIIB complex formation and down-stream signaling activation
About Lyvgen
Lyvgen is an innovative biotech company focused on developing novel therapies for cancer. Its proprietary xLinkAb functional platform generated agonistic antibodies with tumour-localized immunostimulatory activities by balancing multiple functions of candidate antibodies, which has helped to generate a number of drug candidates, including LVGN7409 (CD40 agonist mAb) ,LVGN6051 (4-1BB agonist mAb) and LVGN3616(PD-1 antibody), presently in clinical development. The phase 1 clinical trial of LVGN7409 has been advanced in China and the United States, and preliminary clinical breakthrough safety and activity data have been obtained. The combined PD-1 antibody study is under way. LVGN6051 has demonstrated safety and activity and is being tested in a phase 1B /2 trial in the United States with Merck's PD-1 inhibitor Keytruda® (pembrolizumab) in adults with advanced tumors. LVGN6051 has also been successfully approved in Phase 1B /2 clinical trials in China in combination with small molecule inhibitors of vascular endothelial growth factor receptor, and clinical trials are being actively carried out.
For more information, visit www.lyvgen.com

 沪公网安备 31011502015346号
沪公网安备 31011502015346号